UPMC and Pitt Health Sciences
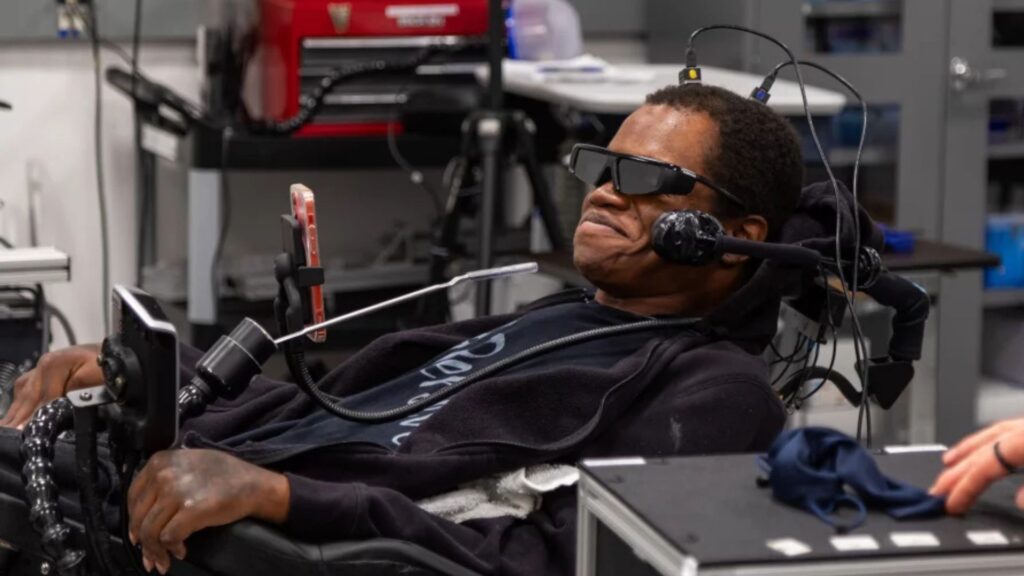
Elon Musk’s Neuralink has announced plans to launch a clinical trial in October 2025 that aims to enable people with speech impairments to translate their thoughts directly into text using brain implants. The U.S. Food and Drug Administration granted Neuralink an Investigational Device Exemption, marking a significant milestone in the expansion of the company’s brain–computer interface (BCI) technology.
Thought-to-Text Trial Details
Neuralink President DJ Seo revealed the trial during a presentation at the Korea Foundation for Advanced Studies in Seoul. Participants will receive Neuralink’s implantable device, which reads neural signals associated with speech planning and converts them into written text in real time. As Seo explained, “If you’re imagining saying something, we would be able to pick that up” (Reuters).
Global Expansion and Ethical Debates
This speech-focused trial follows Neuralink’s first international study at Toronto Western Hospital, where two Canadian quadriplegic patients received implants in late August and early September. The Canadian venture drew criticism from Dr. Raghu Venugopal of University Health Network, who called collaboration with Musk “unethical” due to his support for U.S. domestic policy cuts affecting global health. Conversely, bioethicist Kerry Bowman of the University of Toronto acknowledged the ethical complexity but emphasized the potential life-changing benefits for patients lacking other treatment options (CBC).
Rapid Patient Enrollment and Usage
Neuralink’s trial cohort has grown swiftly from three participants in February to twelve worldwide by September. Collectively, these users have logged over 15,000 hours of device interaction across 2,000 days, demonstrating capabilities such as cursor control, robotic-arm manipulation, and text entry. These early applications underscore the technology’s practical viability for restoring communication and autonomy.
Competitive BCI Landscape
The brain–computer interface sector is heating up. Precision Neuroscience secured FDA clearance for its Layer 7 Cortical Interface, claiming to be the first wireless BCI with regulatory approval. Synchron, supported by investors like Bill Gates and Jeff Bezos, employs a less invasive approach by threading electrodes through blood vessels rather than brain tissue. Neuralink’s direct-implant strategy remains its hallmark for achieving high signal fidelity and bandwidth.
Future Vision Beyond Medical Use
While the current focus is on therapeutic applications, Neuralink envisions expanding to healthy users by 2030, enabling direct, AI-driven communication “at the speed of thought.” CEO Musk and Seo have outlined ambitions to scale implantations to 20,000 per year by 2031, transitioning BCIs from niche medical devices to mainstream consumer technology platforms.
Neuralink’s forthcoming trial represents both a profound leap for assistive neuroscience and a harbinger of ethical, regulatory, and technological debates that will shape the future of human–machine integration.












